IP News, Litigation
Post Grant Review (PGR) Petitions Hitting Orange Book Patents at Higher Rate than Inter Partes Review (IPR)
June 8, 2017
As seen in Law360.
By: Don Prather of Meunier Carlin & Curfman LLC and Kevin Chrustowski of TK Holdings Inc.
According to the U.S. Patent and Trademark Office (USPTO) website as of the end of the government fiscal year 2016, 16%[1] of all Post Grant Review (PGR) petitions have been filed against patents that are currently listed in the FDA Orange Book[2].
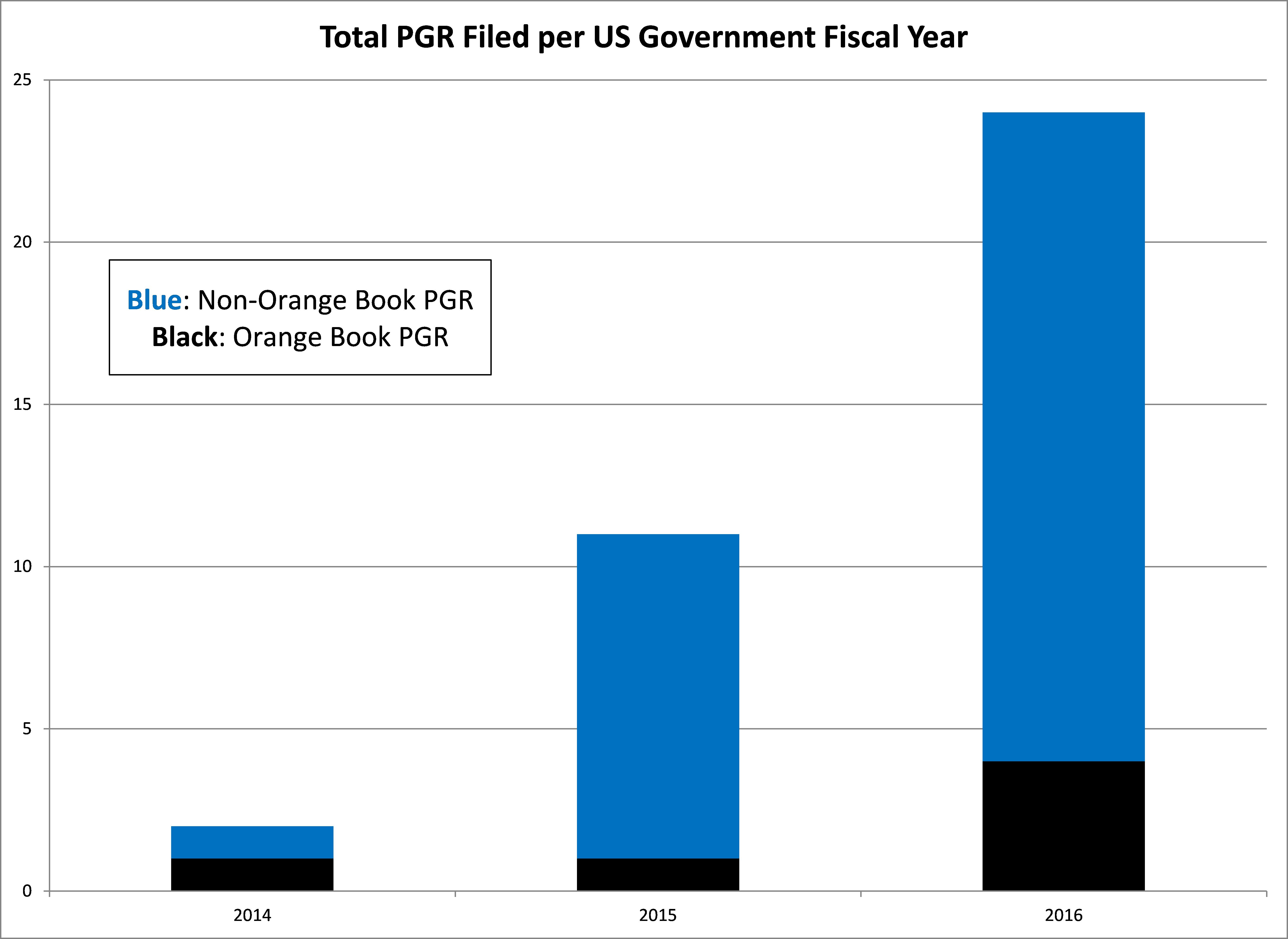
Though the number of PGR petitions is relatively small (37), this could indicate a trend going forward. In contrast, Orange Book patents have been challenged in a much smaller percentage of Inter Partes Review (IPR) petitions, where about 5% of 5143 total cases have been directed to Orange Book listed patents. Considering an IPR petition can be filed against any granted patent, whereas a PGR petition can only be instituted against patents filed on or after the AIA first-inventor-to-file start date of March 16, 2013, it is easy to understand the discrepancy between the overall numbers of IPR and PGR petitions. However, the roughly three-fold greater filing rate against Orange Book patents using PGR attacks should not be ignored.
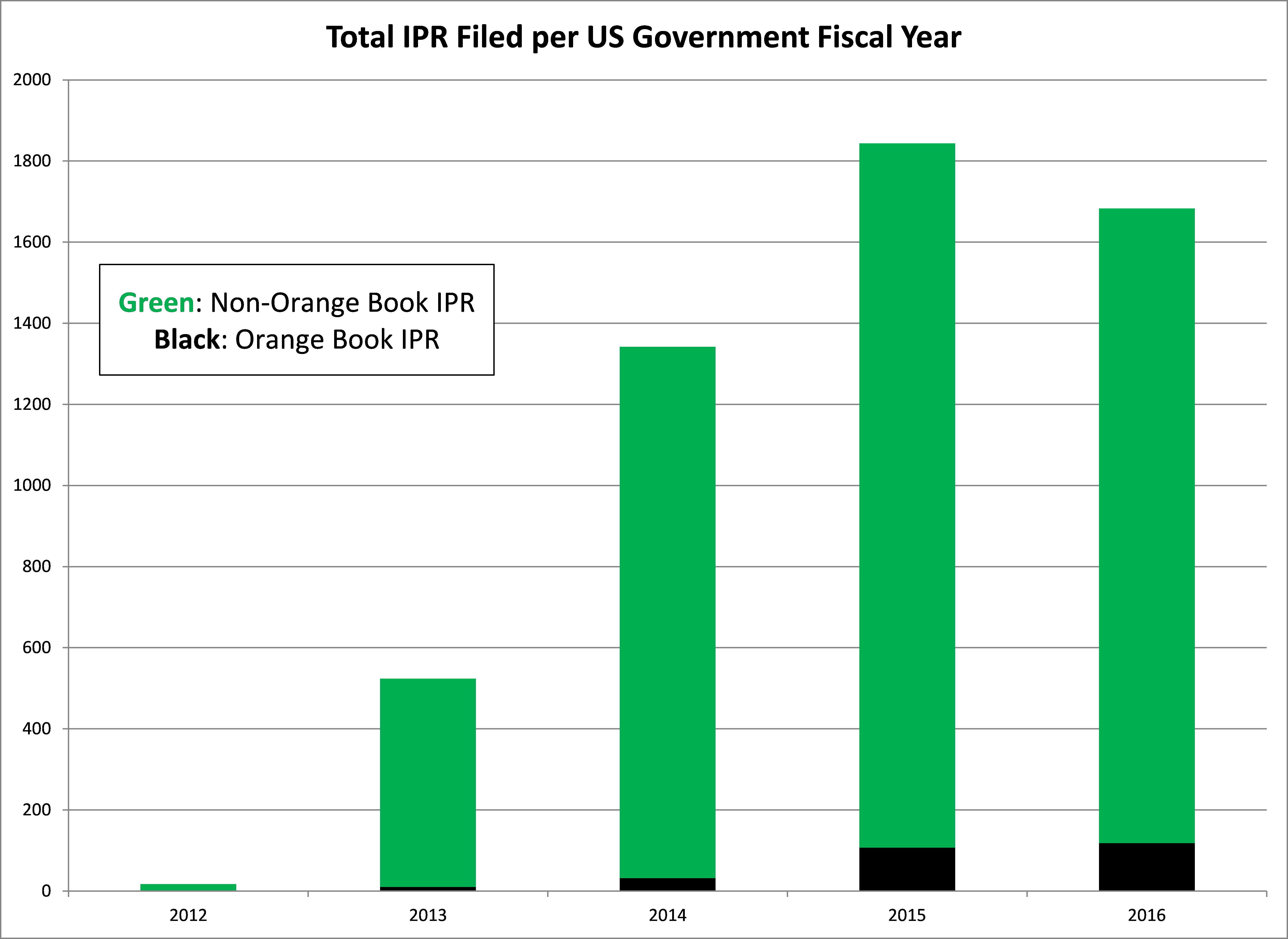
In an analysis of Inter Partes Review (IPR) petitions, only 8% of IPR petitions in FY16 were filed against Orange Book patents, the highest rate of any fiscal year. In contrast, the lowest yearly rate of petitions filed against Orange Book patents in PGR proceedings was 9% in FY15.
The rate of PGR attacks against Orange Book patents in FY14 and FY16 were about 50% and 17%, respectively, of the total PGR petitions in each year. It is clear from these data that PGR attacks on Orange Book patents are much more frequent relative to the total number of PGR petitions, as opposed to IPR petitions.
In addition, the peak of total IPR filings in FY15 includes a large number of petitions filed by non-practicing entities affiliated with hedge funds. In FY15, 27 of the IPR petitions filed against Orange Book patents were filed by the Coalition for Affordable Drugs, which was the primary non-practicing entity filing petitions against Orange Book patents. Therefore, excluding these petitions from these non-practicing entities results in only 5% of all IPRs in FY15 being filed against Orange Book patents. In FY16, two petitions have been filed individually by Kyle Bass and Erich Spangenberg, the two prominent hedge fund managers involved with the IPR process. Thus, excluding these petitions results in only 7% of all IPR petitions in 2016 being filed against Orange Book patents.
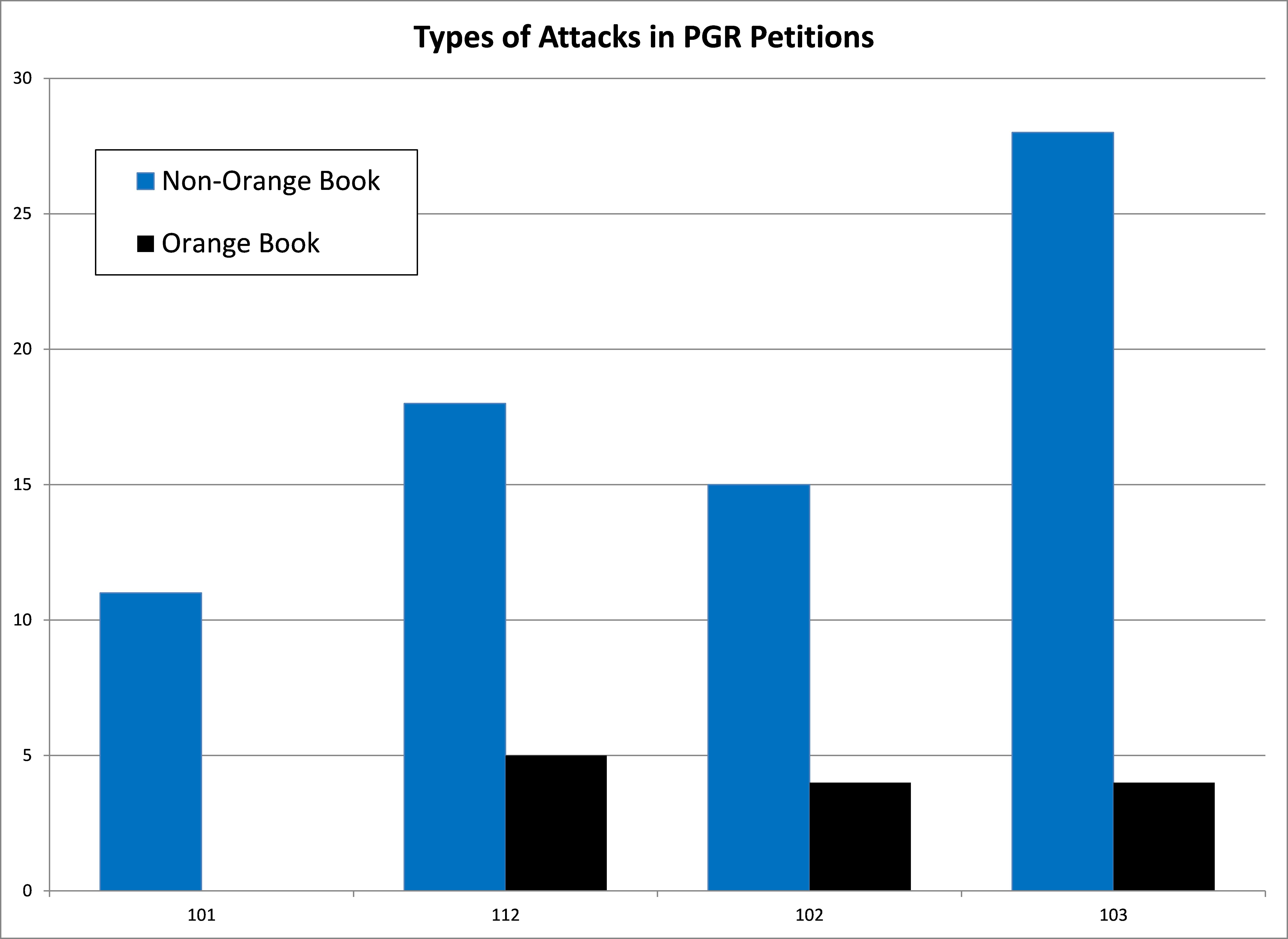
We next examined the invalidity arguments being asserted in the PGR petitions filed against Orange Book patents. The most common form of invalidity attack on Orange Book patents in the PGR petitions was 35 U.S.C. §112, which is not available for IPRs. These attacks were used against each of the 4 Orange Book patents across 6 petitions. Both 35 U.S.C. §102 and §103 were each used against 3 of the patents, whereas there were no petitions that raised invalidity under 35 U.S.C. §101. For the 35 U.S.C. §112 invalidity arguments, a written description argument was used most often, against 3 of the 4 patents, while indefiniteness and enablement were also used. For non-Orange Book patents, PGR petitions are dominated by the use of §103 (90% of petitions) while §112 is used at a much lower rate (58% of petitions). Also of note is that 35% of non-Orange Book petitions raise invalidity arguments under §101, which contrasts sharply with the lack of any invalidity arguments under §101 for Orange Book patents in PGR petitions.
Although the total number of petitions is vastly different between the two new post grant procedures (IPR v. PGR), there appears to be a greater willingness among pharmaceutical companies to wade through the still relatively untested PGR process when compared with their initial hesitation regarding the initiation of IPR proceedings. Perhaps this increased willingness to launch invalidity challenges against Orange Book patents in the PGR process stems from the lower threshold for institution (more likely than not) when compared to IPR (reasonable likelihood of success). A more likely reason for the increased numbers of PGR petitions against Orange Book patents is due to the greater importance of the written description and enablement requirements in the pharmaceutical area, when compared with other fields like electrical or mechanical engineering, where §112 issues are less significant. Together with a heightened indefiniteness standard as laid out by the Supreme Court in Nautilus, Inc. v Biosig Instruments, Inc. (134 S. Ct. 2120 (2014)), pharmaceutical companies can expect increased scrutiny and increased invalidity attacks under §112 in PGR proceedings.
Because the estoppel effect in PGR proceedings extends to all possible invalidity arguments that “reasonably could have been raised,” some companies might choose to wait the nine months and bypass a PGR proceeding in favor of filing an IPR petition, where the estoppel effect would be more limited. However, even with the high success rates of IPR petitions before the Patent Trial and Appeal Board (PTAB), the estoppel concerns raised by the PGR process do not seem to be deterring PGR attacks made on Orange Book patents.
According to the USPTO website, the PTAB has decided only 7 PGRs through FY16, including 1 Orange Book patent. In this initial PGR decision involving an Orange Book patent, the patent claims were upheld. However, if the increased numbers of patent challenges under §112 are successful, this will likely increase the number of new PGR petitions that will hit the PTAB’s docket. With the ever increasing number of post-AIA patents being granted, there are more patents vulnerable to invalidity attacks through PGR proceedings. Thus, the number of PGR petitions is likely to rapidly increase regardless of the specifics of the PTAB decisions. However, the first decisions will likely provide significant insight into the board’s process, assisting petitioners in determining whether the ends justify the means.
In summary, while the number of IPR petitions seems to have stabilized, early indications from the analysis of PGR petitions filed against Orange Book patents show that pharmaceutical companies should be prepared to defend their patent portfolio against an increasing number of PGR petitions.
[1] All percentages are rounded to the nearest whole number.
[2] All Orange Book patents verified at http://www.accessdata.fda.gov/scripts/cder/ob/default.cfm
